Abstract
Background
Unprecedented depth of response is observed with quadruplet combinations in newly diagnosed multiple myeloma (NDMM). The incremental benefit of autologous hematopoietic cell transplantation (AHCT) in this setting has not be described and can be appraised with the serial assessment of minimal residual disease (MRD). Here we describe the impact of AHCT on MM burden assessed by next generation sequencing (NGS) for patients enrolled in the MASTER trial.
Methods
MASTER is a prospective, multi-center clinical trial utilizing daratumumab, carfilzomib, lenalidomide and dexamethasone (Dara-KRd) induction, AHCT (Melphalan conditioning), followed by MRD response-adapted Dara-KRd consolidation with planned enrichment for patients with high-risk chromosome abnormalities (HRCA). MRD assessment is performed by NGS (ClonoSEQ® platform) upon completion of induction therapy with 4 cycles of Dara-KRd, 60-80 days after AHCT and after each 4 cycle-block of consolidation, where applicable. Patients with confirmed MRD negativity (MRD<10 -5 in two consecutive time points) enter treatment free observation and active surveillance of MRD resurgence ("MRD-SURE"). The primary endpoint of the study is negativity utilizing IMWG criteria (MRD<10 −5). Achievement of MRD <10 −6 is an exploratory endpoint. Patients are categorized as having 0, 1, 2+ HRCA [gain 1q, t(4;14), t(14;16), t(14;20), del(17p)]. We describe changes in MRD burden with AHCT and explore patient and disease features influencing magnitude of MRD reduction with AHCT.
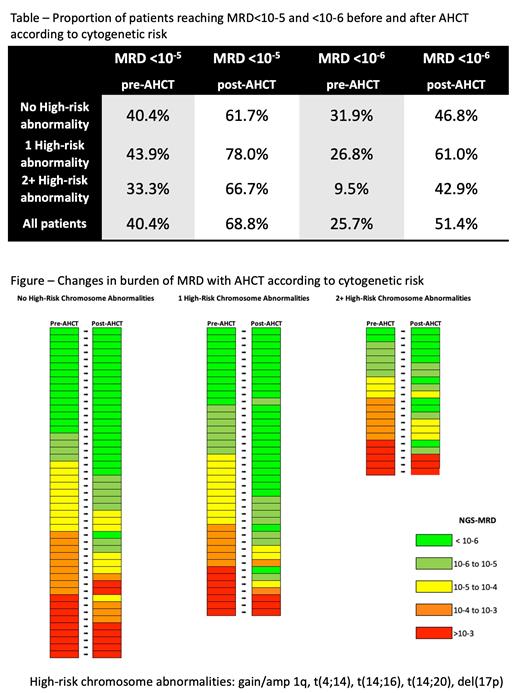
Results
Between 3/2018 and 9/2020, 123 participants were accrued. Of those, 118 were MRD evaluable and 109 have NGS-MRD post-induction and post AHCT and are included in this analysis. The median age is 61 y (35-79) and 18% are 70 or older. Twenty-four percent of patients are non-white, 20% have ECOG 2, 19% high LDH, and 19% R-ISS3. Forty seven (43%) have 0 HRCA, 41 (38%) have 1 HRCA, and 21 (19%) with 2+ HRCA (ultra-HR MM). Forty percent achieved MRD negativity after four cycles of Dara-KRd induction, increasing to 69% after AHCT. Twenty-six percent patients were MRD<10 -6 post induction, increasing to 51% post-AHCT (Table). Of the 65 patients (60%) who remained MRD positive post-induction, 54 (83%) had a reduction in MRD burden with AHCT (figure). The median reduction in MRD with auto-HCT was 1.10 log 10 (range -1.26 to 3.41). Patients with HRCAs had a greater reduction in MRD burden (P=0.02). For patients with 0, 1 and 2+ HRCA, median reduction was 0.91 log 10 (range -0.75 to 2.14), 1.26 log 10 (range -0.21to 3.26) and 1.34 log 10 (range -1.28 to 3.41),respectively. More than 1 log 10 reduction in MRD was seen in 56.0% of patients, 43%, 74% and 71% of patients with 0, 1 and 2+ HRCA respectively. Greater than 2 log reductions in MRD was seen in 20% of patients, 11%, 17% and 43% of patients with 0, 1 and 2 HRCA, respectively. In multivariable analysis that included age, stage, performance status and treatment response post induction, the presence of HRCA was the only factor associated with greater than 1 log 10 reduction in MRD burden with AHCT (OR 3.6, 95% CI 1.27-10.2, P=0.016).
Conclusions
An ultrasensitive quantitative MRD assay using NGS demonstrates the incremental benefit of AHCT in the context of highly efficacious quadruplet induction. The greatest impact is afforded to the highest risk disease subset elucidating the biologic underpinnings of the impact of AHCT in MM. At this time, AHCT should remain an integral part of therapy for fit, NDMM patients, particularly those with the high-risk disease and those who remain MRD positive after induction. Future studies exploring AHCT deferral in NDMM should be focused on patients who are MRD negative post optimal induction.
Chhabra: GSK: Honoraria. Dholaria: Angiocrine: Research Funding; MEI: Research Funding; Pfizer: Research Funding; Jazz: Speakers Bureau; Takeda: Research Funding; Poseida: Research Funding; Janssen: Research Funding; Celgene: Speakers Bureau. Silbermann: Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Sanofi Genzyme: Membership on an entity's Board of Directors or advisory committees, Research Funding. Giri: PackHealth: Research Funding; CareVive: Honoraria, Research Funding. Hari: Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Millenium: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotech: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Karyopharm: Consultancy; Celgene-BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau. Costa: Karyopharm: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal